Loading profile for Chris Doe
Research Interests
Our lab investigates central nervous system development in Drosophila. His lab is currently interested in (1) temporal identity programs used to generate an ordered series of neural progeny from a single progenitor, (2) how spatial patterning and temporal identity are integrated to generate heritable neuronal identity, (3) how neuronal progenitors change competence to respond to intrinsic and extrinsic cues over time, and (4) the developmental mechanisms driving neural circuit assembly, with a focus on larval locomotor circuits and adult central complex circuits.
The role of “temporal transcription factors” in generating an ordered series of neural subtypes
Neuronal diversity is generated in a stepwise fashion. In the first step, neuroectodermal spatial patterning cues (homeodomain proteins expressed in columns and rows) assign a unique identity to every neural progenitor (called neuroblasts in Drosophila). This results in 100 unique neuroblasts in each brain lobe, and 30 unique neuroblasts in each hemisegment of the ventral nerve cord (VNC). The second step of generating neuronal diversity is temporal patterning (the ordered production of different neural subtypes from a single progenitor). Although much is known about spatial patterning mechanisms, relatively little is known about temporal patterning mechanisms.
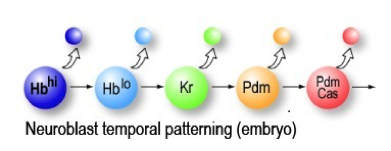
Embryonic neuroblasts sequentially express transcription factors (Hunchback, Krüppel, Pdm, Castor) that specify temporal identity of their neuronal progeny. Isshiki et al., Cell (2001).
There are five “temporal transcription factors” (TTFs) sequentially expressed in embryonic VNC neuroblasts: Hunchback (Ikaros class) > Krüppel (zinc finger class) > Pdm (Pou/homeodomain class) > Castor (Casz1 class) > Grainy head (CP2 class). Each of these factors is sequentially expressed by neuroblasts, and maintained in the neural progeny born during each expression window. Each temporal transcription factor is necessary to specify the neuronal identity produced during its expression window, and early TTFs can suppress the expression of later TTFs to generate ectopic early-born neurons (see Kohwi and Doe, 2014, Nature Reviews Neuroscience).
It is unknown if larval neuroblasts have a similar temporal transcription factor cascade to increase the diversity of neurons in the adult CNS. There are two types of larval neuroblasts: canonical type I neuroblasts bud off progeny called GMCs that differentiate into a pair of neurons, whereas type II neuroblasts produce transit-amplifying cells called intermediate neural progenitors (INPs) that themselves bud off ~6 GMCs to generate ~12 neural progeny. Thus, type II neuroblasts generate much larger, and possibly more complex, cell lineages than type I neuroblasts. Interestingly, type II neuroblasts produce most of the intrinsic neurons of the adult central complex (a brain region with beautiful laminar/columnar organization that is used for multimodal sensorimotor processing).
We are interested in identifying temporal transcription factors in larval type II lineages (both in neuroblasts and in the INPs). We have recently shown that INPs sequentially express Dichaete (Sox family) > Grainyhead > Eyeless (Pax6) transcription factors, and that these temporal transcription factors are required for the production of distinct neural subtypes. Moreover, young type II neuroblasts also transiently express at least three transcription factors and generate different neuronal/glial progeny over time, providing a second temporal identity axis. Thus, neuroblast and INP temporal patterning axes act combinatorially to generate increased neural diversity within the adult brain (Bayraktar and Doe, 2013, Nature). How these two “axes of information” are integrated to generate the specific neurons of the adult central complex remains an open question.
Currently there are many experiments ongoing to identify and functionally characterize both neuroblast and INP transcriptional cascades. For example, we are using TU-tagging (Miller et al., 2009, Nature Methods) to identify novel TTFs during larval type II neuroblast lineages, and to determine their role in assembling the adult central complex. We are also investigating the embryonic origin of type II neuroblasts, to determine (a) if they form as type II neuroblasts “de novo” or by transition from a simpler type I neuroblast; (b) if they use the Hunchback>Kruppel>Pdm>Cas>Grh cascade of TTFs during their embryonic lineages, and if these factors are maintained in INP lineages; and (c) to identify the neurons produced by embryonic type II neuroblasts and determine if they are the pioneers of the adult central complex neuropil.
The integration of spatial and temporal patterning to generate heritable neuronal identity
Spatial patterning to specify neuroblast identity occurs in the neuroectoderm, just prior to neuroblast delamination and initiation of its cell lineage. Yet these transient spatial patterning cues somehow generate a heritable cell fate that is maintained by neuroblasts cultured in isolation or transplanted into a new spatial location within the embryo. It remains unknown how transient spatial cues lead to heritable neuroblast identity. Even more interesting, the spatial cues that specify unique neuroblast identity must be combined with the sequential expression of temporal transcription factors to generate lineage-specific cell fates. For example, NB7-1 is specified by the combination of Engrailed (row 6/7) and Ventral nervous system defective (Vnd; row 1); the first temporal transcription factor Hunchback induces a motor neuron identity (U1) in this lineage. In contrast, NB7-2 has a different spatial code: Engrailed (row 6/7) and Intermediate nervous system defective (Ind; row 2) and the first temporal transcription factor Hunchback induces an interneuron identity in this lineage. How do the spatial patterning genes shift the output of Hunchback from making a motor neuron in one lineage (NB7-1) and an interneuron in the adjacent lineage (NB7-2)? Or a serotonergic neuron in the next adjacent lineage (NB7-3)?
The question of how transient spatial patterning cues impart a heritable neuroblast identity, and how this neuroblast identity “flavors” the output of subsequent temporal transcription factors, are two of the most important open questions for understanding the generation of neuronal identity.
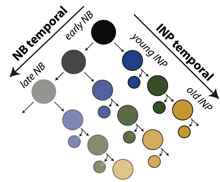
Larval type II neuroblasts undergo changes in gene expression that indicate temporal patterning, while their intermediate neural progenitors (INPs) sequentially express three transcription factors (Dichaete, Grainyhead, Eyeless). The combination of neuroblast and INP temporal identity axes increases the neural diversity generated by a single progenitor.
The development and function of locomotor circuits
Over the past 30 years we have learned a great deal about the specification and connectivity of motor neurons to their target body wall muscles. To determine how these motor neurons are used to generate larval locomotion it is essential to identify the interneurons in the locomotor circuits. Yet almost nothing is known about interneuron specification (just a few exemplar interneurons have been characterized, out of the ~270 interneurons per hemisegment of the VNC), and even less is known about interneuron function in locomotor behavior.
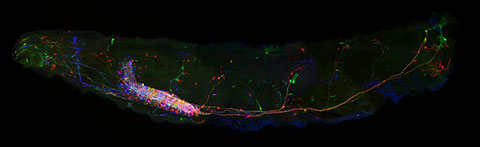
Confocal image of an entire Drosophila larva stained using the Multicolor Flip Out (MCFO) method of Nern et al. (2015) PNAS 112:E2967. Note that staining the intact larva required development of a novel fixation method (Manning and Doe, unpublished) Image: Laurina Manning.
To initiate a comprehensive analysis of interneuron diversity, including their role in locomotion, we have identified several hundred Gal4 lines expressed in 1–5 interneurons (Manning et al., 2012, Cell Reports). We have used these lines in two ways. First, we have mapped them into a three-dimensional atlas that allows us to uniquely identify more than 50 percent of all interneurons in the ventral CNS (Heckscher et al., 2014, Development), and are currently linking each neuron to its developmental origin by adding lineage and TTF markers to the atlas. Second, we are using these lines to screen the interneurons for a role in larval behavior. We have expressed the warmth-activated TrpA1 channel in each “sparse interneuron Gal4 line” and found lines where neuronal activation leads to behavioral defects such as reverse locomotion, turning only, feeding only, left-right uncoordinated locomotion, pausing, and rigid paralysis (Matt Clark, submitted). We have investigated one phenotype (left-right uncoordination), showing that the affected interneurons are Even-skipped (Eve)+ contralateral ascending interneurons (that are conserved in mouse; Evx1/2). We used thermogenetics and optogenetics to determine the function of these interneurons in larval locomotion and used a TEM (transmission electron microscopy) serial reconstruction of the entire larval CNS to identify pre- and postsynaptic partners to define a proprioceptive sensorimotor circuit (Heckscher et al., 2015, Neuron).
The characterization of interneurons in other phenotypic categories remains to be studied. In addition, we are using the circuits we identify as an entry point for testing hypotheses for how interneurons and motor neurons assemble locomotor circuits: common transcriptional programs, common birth order, or common lineage. Future directions include studying plasticity and compensation within these circuits, as well as their remodeling and participation in adult locomotor circuits.
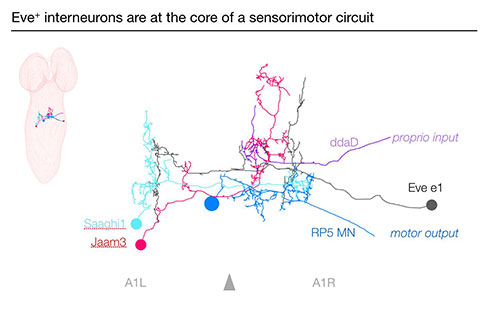
Neural connectivity linking proprioception (ddaD neuron) to motor output (RP5 neuron) in the larval CNS. The Even-skipped+ interneurons (e.g. Eve e1, shown) are responsible for maintaining left/right balanced motor output during forward locomotion. See: Heckscher ES, Zarin AA, Faumont S, Clark MQ, Manning L, Fushiki A, Schneider-Mizell CM, Fetter RD, Truman JW, Zwart MF, Landgraf M, Cardona A, Lockery SR, Doe CQ (2015) Even-skipped+interneurons are core components of a sensorimotor circuit that maintains left-right symmetric muscle contraction amplitude. Neuron, 88, 314-329.
Recent publications
(pulled from pubmed)