Home | About | Faculty | Calendar | Facilities | Graduate program | Contact | Apply
This page is optimized for viewing with javascript.
Victoria DeRose

Professor, Chemistry and Biochemistry
Associate Member, IMB
Ph.D., University of California, Berkeley
B.A., University of Chicago
Email
Lab website
Office: 355A Klamath Hall
Office Phone: 541-346-3568
Lab: Onyx Bridge Room 270
Lab Phone: 541-346-4613
Loading profile for Victoria DeRose
Research Interests
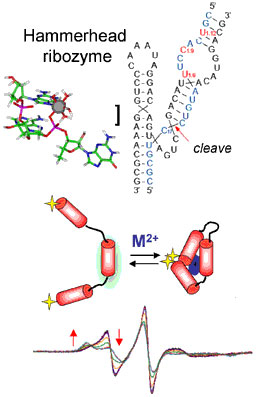
We are investigating chemical activity and structure in nucleic acids and proteins, with an emphasis on metal interactions. Proteins have long been known to exploit and tune the reactive properties of metals in order to perform reactions that are sometimes unavailable to the benchtop chemist. It has only recently been determined that ribonucleic acid (RNA) also catalyzes chemical reactions in certain biologically important systems. RNA has its own distinctive metallobiochemistry. Our research group examines such systems using tools of biological and bioinorganic chemistry, and spectroscopic methods. These are interdisciplinary studies that lie at the interface of biology and chemistry.
RNA is a truly unique biopolymer that displays a rich array of cellular functions, some of which are still being uncovered. RNA structure itself is complex and dynamic, and can be profoundly influenced by ionic conditions. One long-term objective of our research program is to directly measure cation-RNA interactions and understand their importance in function. Catalytic RNAs, or ribozymes, provide model systems for these studies. Since their discovery approximately two decades ago, the mechanisms by which RNA catalyzes reactions have been an area of intense investigation. Biological ribozymes catalyze phosphoryl transfer reactions in RNA processing and splicing events. A growing body of evidence indicates that the aminoacyl transferase activity of the ribosome also is catalyzed in an RNA-formed active site. Cations influence activity in these systems by mechanisms that are not entirely understood, but range from general electrostatic effects to population of very specific ‘sites’ created by the folded RNA. Our current projects include detailed studies of ribozymes such as the hammerhead motif derived from the genomes of plant viroids and other organisms. We are also initiating an investigation of the interactions of metal-based therapeutics, such as the anticancer compound cisplatin, with structured RNAs.
In the active sites of metalloenzymes, the properties of metal ions are highly tuned by their protein environments. In order to understand the importance of different ‘spheres of influence’ that the protein exerts on the metal ion, we are designing and investigating small peptides based on the metal-binding cavities of naturally-occurring enzymes. The active sites of blue copper proteins and of mononuclear Fe and Co-containing enzymes are current targets using both rational and combinatorial methods to create appropriate peptide models.
These studies require spectroscopic techniques that examine global structure as well as provide a window of observation around the metal ion. EPR, NMR, fluorescence, and other spectroscopic methods are used in these projects. SDSL (site-directed spin labeling) allows tracking of RNA structure by monitoring changes in local dynamics and interprobe distances. ENDOR (electron nuclear double resonance) spectroscopy is a double resonance technique that detects only nuclei that are coupled to a paramagnetic metal ion. When combined, these methods provide unique information about global structure and local environments in the active sites of metalloproteins and ribozymes.
Recent publications
(pulled from pubmed)
Recent publications
(pulled from pubmed)
Moghaddam AD, White JD, Cunningham RM, Loes AN, Haley MM, DeRose VJ
Dalton Trans 2015 Feb 28;44(8):3536-9
White JD, Guzman LE, Zakharov LN, Haley MM, DeRose VJ
Angew Chem Int Ed Engl 2015 Jan 12;54(3):1032-5
Ward WL, Plakos K, DeRose VJ
Chem Rev 2014 Apr 23;114(8):4318-42
Osborn MF, White JD, Haley MM, DeRose VJ
ACS Chem Biol 2014 Oct 17;9(10):2404-11
White JD, Osborn MF, Moghaddam AD, Guzman LE, Haley MM, DeRose VJ
J Am Chem Soc 2013 Aug 14;135(32):11680-3
Hostetter AA, Osborn MF, DeRose VJ
ACS Chem Biol 2012 Jan 20;7(1):218-25
Chapman EG, DeRose VJ
J Am Chem Soc 2012 Jan 11;134(1):256-62
Ward WL, Derose VJ
RNA 2012 Jan;18(1):16-23
Hostetter AA, Miranda ML, DeRose VJ, McFarlane Holman KL
J Biol Inorg Chem 2011 Dec;16(8):1177-85
Chapman EG, Hostetter AA, Osborn MF, Miller AL, DeRose VJ
Met Ions Life Sci 2011;9:347-77
Chapman EG, DeRose VJ
J Am Chem Soc 2010 Feb 17;132(6):1946-52
Hostetter AA, Chapman EG, DeRose VJ
J Am Chem Soc 2009 Jul 8;131(26):9250-7