Home | About | Faculty | Calendar | Facilities | Graduate program | Contact | Apply
This page is optimized for viewing with javascript.
Mike Harms

Assistant Professor, Chemistry and Biochemistry
Member, IMB
Ph.D. Johns Hopkins University
B.S. Oregon State University
Email
Lab website
Office: Willamette Hall Room 340A
Office Phone: 541-346-9002
Lab: Willamette Hall Room 340
Lab Phone: 541-346-9003
Loading profile for Daniel Grimes
Research Interests
We study how symmetries are broken and maintained during embryonic development and growth. Why are our hearts on the left and how do they come to be there? How do our two arms develop to be the same size? How does our spine stay straight during growth?
These interests have led us into all kinds of fascinating areas of biology including cilia and fluid flow in the embryo and brain, the transduction of mechanical signals, the enigmatic Polycystin proteins, ciliated neurons, cell polarity, and more.
We use a variety of procedures to address these questions including genetics, single cell sequencing, confocal imaging and micro-computed tomography. We primarily use the zebrafish model organism.
Our recent work addresses the following questions:
How is embryonic symmetry broken?
In many vertebrates, early embryonic left-right symmetry is broken by an asymmetrical fluid flow generated by beating cilia (reviewed in Grimes and Burdine, Trends in Genetics, 2017). Sensation of that flow - which also depends on cilia - requires Polycystin family transmembrane proteins ( Grimes et al., Plos Genetics, 2016). We study the pathways by which flow signals are transduced and ask how these pathways control the emergence of left-right asymmetries in the embryo.
How does the spine retain symmetry?
The spine is the defining feature of vertebrate life, but how it remains straight during growth is a mystery. In zebrafish mutants that lack cilia motility, cerebrospinal fluid flow is compromised. This results in late-onset spinal curves that appear during a growth spurt (Grimes et al., Science, 2016). These mutants closely model the human disease idiopathic scoliosis, the presence of abnormal 3D spinal curves that often occurs in adolescence. We apply micro-computed tomography, CRISPR mutagenesis, and single cell techniques to understand the roles of cilia and flows in maintaining spinal symmetry and to model idiopathic scoliosis.
Figures:
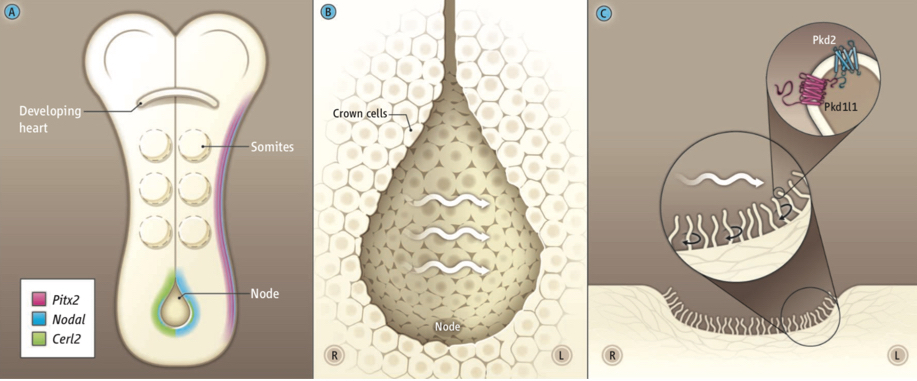
Left-right patterning of the embryo depends on an asymmetrical
cilia-driven fluid flow that occurs transiently in a midline structure
called the Left-Right Organizer (the node in mouse, Kupffer's vesicle in
zebrafish). Motile cilia within the node drive asymmetric flow which is
sensed by the embryo in an elusive mechanism that depends on the
transmembrane Polycystin proteins PKD1L1 and PKD2. This sensory pathway
ultimately results in activation of the Nodal cascade on only the left
side of the embryo. Image taken from
Norris
and Grimes 2012, Science.
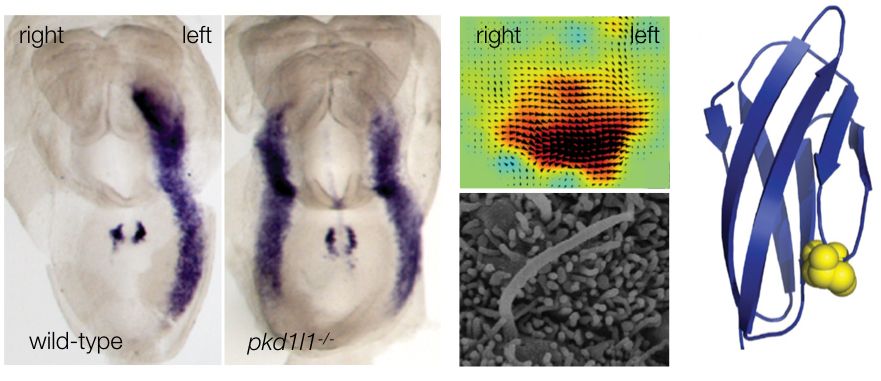
Left panels: While the wild-type embryo shows Nodal expression on the left side, mutants lacking the Polycystin protein PKD1L1 exhibit bilateral Nodal expression. This implicates PKD1L1 in the breaking of embryonic left-right symmetry. Center panels: Motile cilia in the embryonic node generate asymmetric fluid flow. The upper figure shows flow vectors in the node that demonstrate strong leftward flow, while the lower figure shows a scanning electron micrograph of one of the motile cilia that generate this asymmetric flow. Right panel: a structural model of an extracellular domain of PKD1L1 that we have implicated in the transduction of flow-based signals. A critical aspartic acid residue is highlighted which, when mutated, results in the protein losing its sensitivity to flow and causes left-right organ patterning defects in the mouse embryo. Images are taken from Grimes et al., 2016, PLoS Genetics and Field et al., 2011, Development .
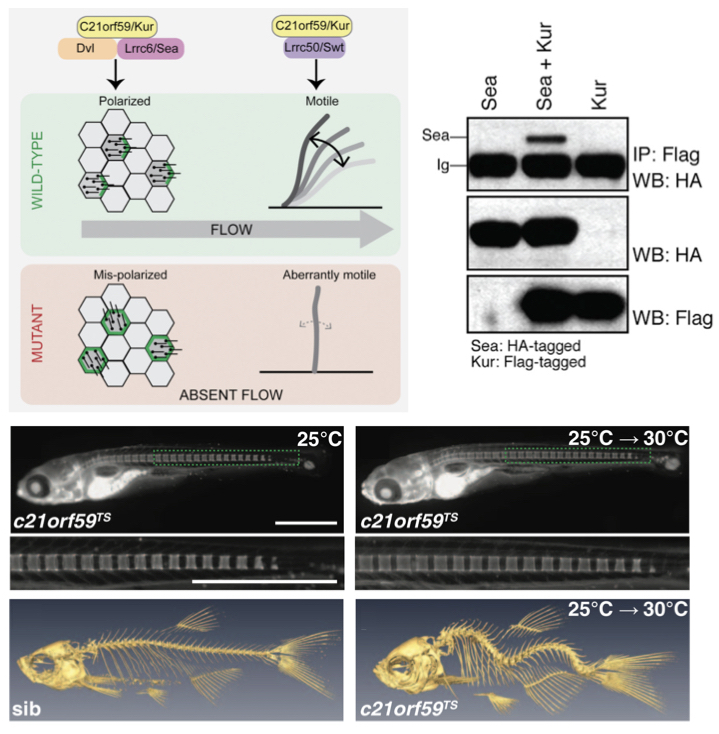
Upper panels: We found that the protein C21ORF59, also called Kurly (Kur), has a dual role in cilia biology. First, it is essential for cilia motility and makes interactions with LRRC50/Swt; both proteins are required for loading the axonemal dynein arms that power cilia beating. Second, C21ORF59 is also required for correct cilia polarity and interacts with components of the planar cell polarity pathway including DVL; in it's absence, cilia do not point in the correct direction. The absence of cilia motility and polarization in c21orf59 mutants results in loss of cilia-generated fluid flows. Lower panels: By investigating a temperature-sensitive (TS) c21orf59 mutant, we found that late-onset spinal curves form in zebrafish lacking cilia motility, even though vertebrae are correctly spaced and patterned. These mutants closely model the common human disease idiopathic scoliosis. Figures taken from Jaffe et al., 2016 Cell Reports and Grimes et al., 2016 Science .
Recent publications
(pulled from pubmed)
Recent publications
(pulled from pubmed)
Harms MJ, Thornton JW
Nature 2014 Aug 14;512(7513):203-7
Hart KM, Harms MJ, Schmidt BH, Elya C, Thornton JW, Marqusee S
PLoS Biol 2014 Nov;12(11):e1001994
Harms MJ, Eick GN, Goswami D, Colucci JK, Griffin PR, Ortlund EA, Thornton JW
Proc Natl Acad Sci U S A 2013 Jul 9;110(28):11475-80
Harms MJ, Thornton JW
Nat Rev Genet 2013 Aug;14(8):559-71
Eick GN, Colucci JK, Harms MJ, Ortlund EA, Thornton JW
PLoS Genet 2012;8(11):e1003072
Harms MJ, Schlessman JL, Sue GR, García-Moreno B
Proc Natl Acad Sci U S A 2011 Nov 22;108(47):18954-9
Harms MJ, Thornton JW
Curr Opin Struct Biol 2010 Jun;20(3):360-6
Bridgham JT, Eick GN, Larroux C, Deshpande K, Harms MJ, Gauthier ME, Ortlund EA, Degnan BM, Thornton JW
PLoS Biol 2010 Oct 5;8(10)
Harms MJ, Castañeda CA, Schlessman JL, Sue GR, Isom DG, Cannon BR, García-Moreno E B
J Mol Biol 2009 May 29;389(1):34-47
Harms MJ, Schlessman JL, Chimenti MS, Sue GR, Damjanović A, García-Moreno B
Protein Sci 2008 May;17(5):833-45
Harms MJ, Wilmarth PA, Kapfer DM, Steel EA, David LL, Bächinger HP, Lampi KJ
Protein Sci 2004 Mar;13(3):678-86