Home | About | Faculty | Calendar | Facilities | Graduate program | Contact | Apply
This page is optimized for viewing with javascript.
Annie Powell

Assistant professor, Biology
Member, IMB
Ph.D., Oregon Health and Science University
B.A., Concordia University
Email
Office: 218 Streisinger Hall
Office Phone: 541-346-2403
Lab: 215A-C Streisinger Hall
Lab Phone: 541-346-2065
Loading profile for Annie Powell
Research Interests
Research in the Powell lab focuses on small intestinal and colonic epithelial stem cell behavior. The intestinal epithelium is a highly dynamic tissue. Normal homeostasis requires intricate control of stem and progenitor cell proliferation, differentiation, migration and ultimately death and detachment. There are several unique populations of progenitor cells within the colonic crypt, and it is posited that each has a unique ability to differentially regulate intestinal homeostasis. These populations are marked by a number of different proteins and have different proliferative properties. The dynamic nature of these intestinal stem cells is likely exploited in normal process of development or repair after injury, but also in malignant transformation.
Our previous research has shown that Leucine rich repeats and immunoglobulin-like domains 1 (Lrig1), a pan-ErbB negative regulator, marks a distinct population of largely quiescent intestinal stem cells and the protein functions as a growth repressor. These discoveries about the Lrig1+ stem cell population tell us something about the role of the cells within the normal small intestinal and colonic crypts in regulating powerful growth factor signaling cascades, as well as inform us about the potential of both Lrig1 and Lrig1-expressing cells in epithelial repair and regeneration after injury. It is unknown how manipulation of one stem cell population affects the colonic tissue repair process in other stem or progenitor populations. Some fundamental questions we address are:
- What requirement, if any, is there for stem cells in tissue regeneration after injury?
- What role do negative regulators, like Lrig1, play in this repair process?
- How do the components of the developing stem cell niche- the epithelium and microenvironment- shape the formation of the adult gut stem cell niche?
The general approach of our research is to apply sophisticated mouse modeling with ex vivo crypt manipulations (organoid culture) coupled with high, or super-resolution microscopy and large-scale genomic scans to address the fundamental questions proposed above. Our studies take advantage of our unique ability to engineer cellular changes within the niche and directly examine effects on the stem cells and influences downstream on the niche. By employing both in vivo and ex vivo approaches, we hope to decipher the molecular cues important for intestinal stem cell maintenance and how these cues may go awry in disease states, such as inflammatory bowel disease and cancer.
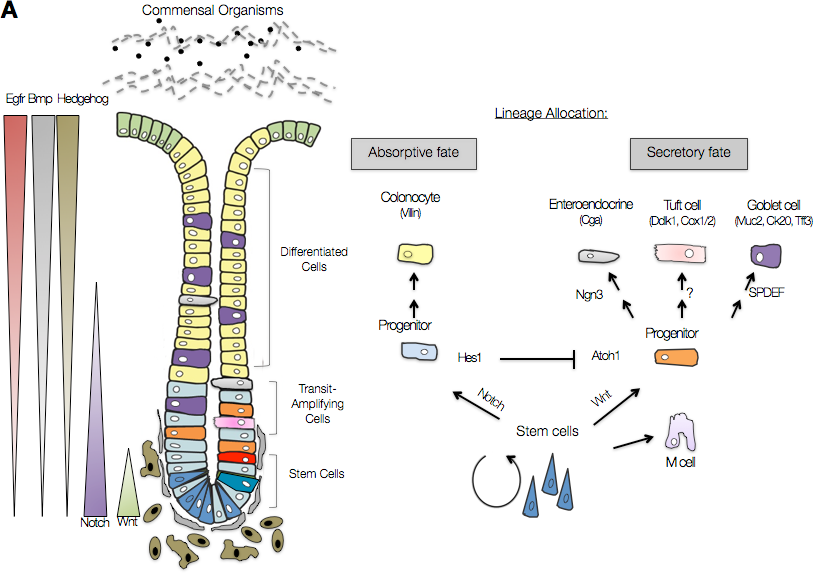
The colonic epithelium (stem, transit-amplifying and differentiated cells) is dependent upon proper regulation of signaling cascades, which direct cell division, migration and maintenance. There is cross-talk between the stromal cells (brown) and the epithelium. Together, the stromal cells and the epithelium achieve proper barrier function. Colors in crypt illustration (left) coordinate with the lineage tree (right).
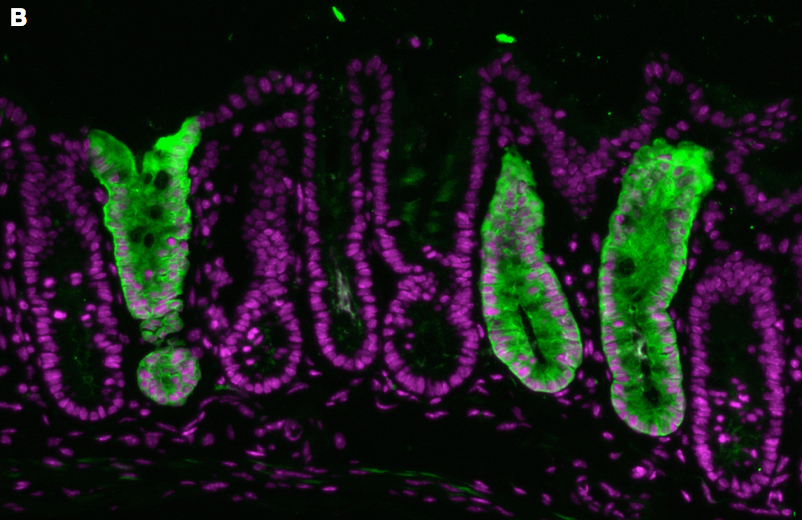
Cross-section of a distal portion of a mouse colon from an induced Lrig1-CreERT2/+;R26R-YFP mouse. U-shaped crypts are made of a single layer of epithelial cells, surrounded by supporting cells. In this lineage labeling experiment, some crypts have undergone genetic recombination to reveal daughter cells of Lrig1-expressing progenitors. These crypts express yellow fluorescent protein (green). All cells in the image are labeled with DAPI (purple).
Recent publications
(pulled from pubmed)
Recent publications
(pulled from pubmed)
Kondo J, Powell AE, Wang Y, Musser MA, Southard-Smith EM, Franklin JL, Coffey RJ
Gastroenterology 2015 Aug;149(2):407-419.e8
Powell AE, Vlacich G, Zhao ZY, McKinley ET, Washington MK, Manning HC, Coffey RJ
Am J Physiol Gastrointest Liver Physiol 2014 Jul 1;307(1):G16-23
Poulin EJ, Powell AE, Wang Y, Li Y, Franklin JL, Coffey RJ
Stem Cell Res 2014 Nov;13(3 Pt A):422-30
Noto JM, Khizanishvili T, Chaturvedi R, Piazuelo MB, Romero-Gallo J, Delgado AG, Khurana SS, Sierra JC, Krishna US, Suarez G, Powell AE, Goldenring JR, Coffey RJ, Yang VW, Correa P, Mills JC, Wilson KT, Peek RM Jr
PLoS One 2013;8(1):e54344
Washington MK, Powell AE, Sullivan R, Sundberg JP, Wright N, Coffey RJ, Dove WF
Gastroenterology 2013 Apr;144(4):705-17
Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ
Cell 2012 Mar 30;149(1):146-58
Powell AE, Anderson EC, Davies PS, Silk AD, Pelz C, Impey S, Wong MH
Cancer Res 2011 Feb 15;71(4):1497-505
Powell AE, Shung CY, Saylor KW, Müllendorff KA, Weiss JB, Wong MH
Stem Cell Res 2010 Jan;4(1):3-9
Levin TG, Powell AE, Davies PS, Silk AD, Dismuke AD, Anderson EC, Swain JR, Wong MH
Gastroenterology 2010 Dec;139(6):2072-2082.e5
Davies PS, Powell AE, Swain JR, Wong MH
PLoS One 2009 Aug 6;4(8):e6530
Davies PS, Dismuke AD, Powell AE, Carroll KH, Wong MH
BMC Gastroenterol 2008 Dec 2;8:57